

Lab Whole Process Solution
Laboratory Quality Control Management
Value Embodiment
The Laboratory Full-Process Solution addresses the managerial challenges of "data quality control, personnel quality control, process quality control, and material quality control" in laboratories by overseeing the five key aspects of "personnel, equipment, materials, methods, and environment."
Personnel & Methods (SOP): Real-time recording of sample verification and SOP-compliant operations through a comprehensive quality control system.SOP-guided process control ensures standardized operations with real-time documentation, integrating smart devices like time-lapse incubators to manage and analyze patient data, embryo information, personnel performance, and outcome-related data.
Equipment & Environment: A fully connected laboratory monitoring system utilizes various sensors deployed in key equipment and environments to maintain real-time awareness of their status. It promptly alerts in case of abnormalities and integrates patient information for enhanced equipment analysis and management.
Materials: Comprehensive management of gametes, embryos, and working materials in the laboratory through embryo bank and reagent/ consumable management systems, seamlessly linking with overall diagnostic and treatment information.
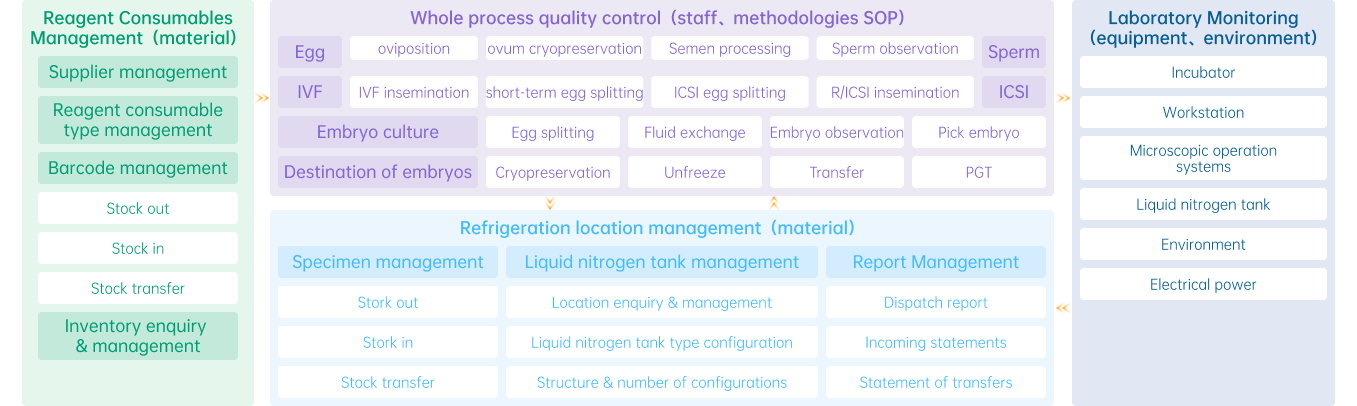
Comprehensive Quality Control (QC) System
The Comprehensive QC System is a workflow system tailored specifically to the detailed operational steps and contents of laboratories.
Leveraging RFID technology and mobile devices, it efficiently and automatically accomplishes data collection and verification while simultaneously facilitating process-based management and data analysis, thereby realizing comprehensive quality control over the entire laboratory operation process.
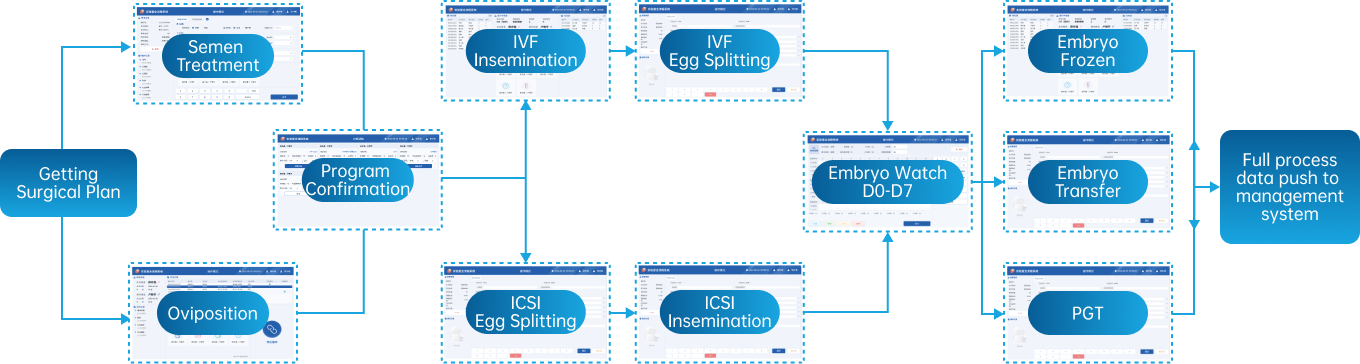
Laboratory Monitoring System
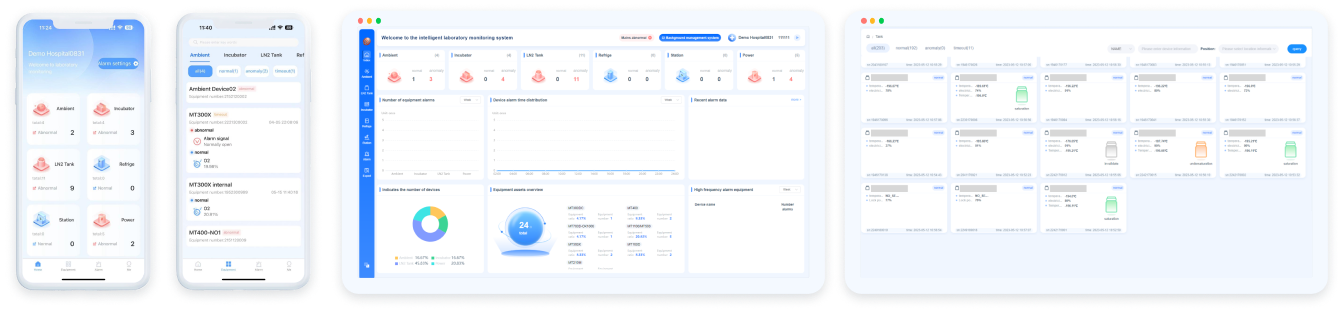
The Laboratory Monitoring System is an intelligent system that encompasses comprehensive surveillance and management of critical equipment types and environments across the entire laboratory. It not only ensures the operational safety of laboratory equipment and environments but also, within the holistic laboratory process solution, integrates patient information to provide crucial data on equipment and environmental conditions for quality control analysis. This data aids in identifying the causes of data fluctuations and efficiently facilitates equipment management, among other functions.
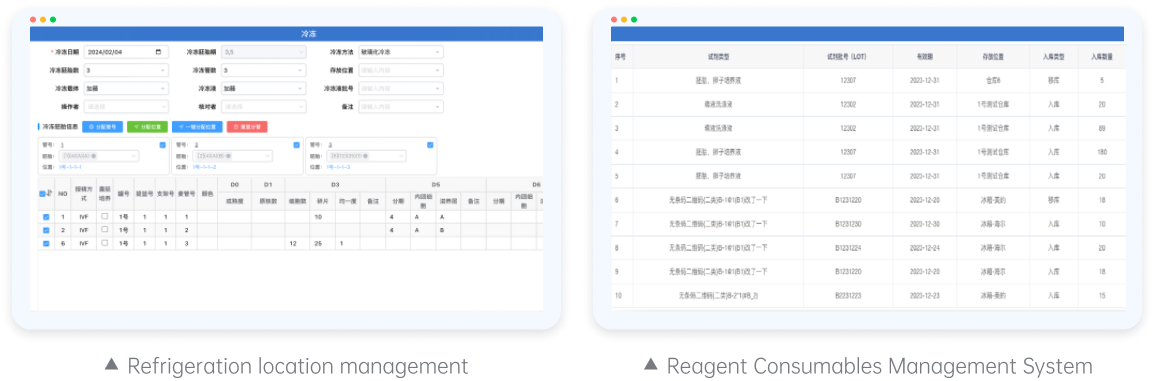
Cryogenic Position Management and Reagent/Consumable Materials Management System
The Cryogenic Position Management and Reagent/Consumable Materials Management System serves as a vital tool for managing essential laboratory assets (such as gametes and embryos) and operational materials (reagents and consumables). Beyond facilitating efficient inventory tracking and management of cryogenic samples and reagent/consumable materials for fertility centers, this system, when integrated with patient information within the comprehensive laboratory process solution, simplifies management and quality control analysis effortlessly.

Contact Us
Telephone:027-85513782
Email:marketing@huchuang.com
Address:3rd Floor, Building 5, Fenghuo Innovation Valley, Wuhan Academy of Postal Sciences, Hubei Province